1. A Review of General Chemistry
Formal Charges
1. A Review of General Chemistry
Formal Charges - Video Tutorials & Practice Problems
On a tight schedule?
Get a 10 bullets summary of the topicWe use our knowledge of valance electrons to determine what he formal charge of the molecule will be.
Formal and Net Charge
1
concept
Calculating formal and net charge.
Video duration:
1mPlay a video:
Easiest formal charge formula you will find anywhere on the internet:
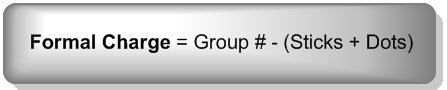
The Net Charge is the sum of all the formal charges on a molecule.
Calculate the formal charge of the following molecule:
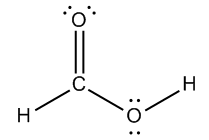
2
concept
Calculate the formal charges of ALL atoms.
Video duration:
2mPlay a video:
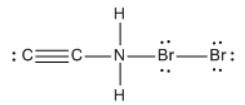
3
Problem
ProblemCalculate the net charge of the molecule

A
-1
B
0
C
+1
D
+2
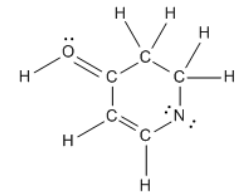
4
Problem
ProblemCalculate the total charge of the molecule

A
-2
B
-1
C
0
D
+1
Hell yeah! That’s literally all there is to know. Don’t let any professors complicate it more for you.
Do you want more practice?
We have more practice problems on Formal Charges
Additional resources for Formal Charges
PRACTICE PROBLEMS AND ACTIVITIES (21)
- An atom with a formal charge does not necessarily have more or less electron density than the atoms in the mol...
- An atom with a formal charge does not necessarily have more or less electron density than the atoms in the mol...
- LOOKING AHEAD In Chapter 3, we describe how the formal charge on an atom can be used to predict the number of ...
- (••) By moving an electron pair, draw a better Lewis structure that minimizes formal charges. (b)
- (••) By moving an electron pair, draw a better Lewis structure that minimizes formal charges. (a)
- Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confir...
- Calculate the formal charge of the indicated atom in the following molecules or ions. (f)
- Calculate the formal charge of the indicated atom in the following molecules or ions. (e)
- Draw the Lewis structure for the following molecules. Be sure to calculate the formal charge of each atom as a...
- Draw the Lewis structure for the following molecules. Be sure to calculate the formal charge of each atom as a...
- Calculate the formal charge of the indicated atom in the following molecules or ions. (d)
- Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confir...
- Draw the Lewis structure for the following ions. Be sure to calculate the formal charge of each atom to confir...
- (a) Calculate the formal charge of the indicated atoms in the ions shown.
- Calculate the formal charge on the non-hydrogen atoms in the molecules shown. Use the arrow-pushing formalism ...
- LOOKING AHEAD In Chapter 3, we describe how the formal charge on an atom can be used to predict the number of ...
- Based on the formal charge, determine how many lone pairs are on each indicated atom. (a)
- Give each atom the appropriate formal charge:a. <IMAGE>b. <IMAGE>
- Draw the missing lone-pair electrons and assign the missing formal charges for the following:c. <IMAGE>d...
- Give each atom the appropriate formal charge:c. <IMAGE>d. <IMAGE>
- Draw the missing lone-pair electrons and assign the missing formal charges for the following:a. <IMAGE>b...

