Alright, guys. Now we're going to lead off this section talking about conformers. And conformers are kind of the big umbrella under which a lot of different topics fall under. For example, if you've heard of Newman projections or chair flips or anything like that, all of that has to do with conformational changes. So in order for us to understand those really important topics, we're going to need to understand what a conformer is first. So let's go ahead and do that.
Most organic molecules have the ability to exist in multiple arrangements without experiencing any chemical changes. And the reason for this is because many of these, single bonds or sigma bonds, are able to rotate. Basically, sigma bonds, if you remember, are those single bonds that have one region of overlap. So it's very easy for one of the atoms to rotate over and over without actually changing the strength or the identity of that bond. What that means is that as that atom rotates, that is not going to be an isomer because it's not actually changing the actual connectivity of the atom. It's still connected the same exact way, it's just rotated a little bit. Structurally, the molecule is never going to change.
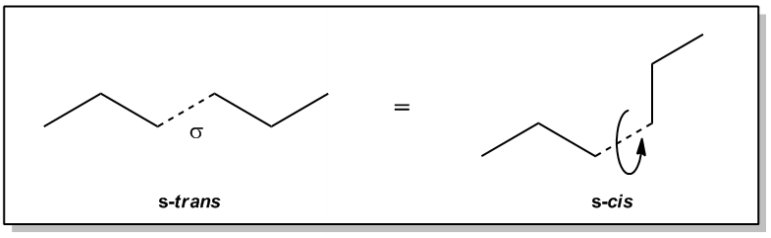
Let me show you an example of hexane. Now, hexane would be a 6-chain and as you can see what I've done here is I've made a little dotted bond with a sigma symbol. This is just a regular sigma bond that I want to show you how it can rotate. So if I were to take this bond and analyze, if this was a double bond, would it be cis or trans? What we would do is we would draw our fence and we would say that the big groups are on different sides. So this would be trans. And the thing is that single bonds don't have the ability to stay locked in place because, remember, a double bond, once it's trans, it's going to stay trans forever. Or once it's cis, it's going to stay cis forever. But single bonds are able to freely rotate from these transpositions to the cispositions easily. Instead of calling this trans and cis, it's the conformation of a sigma bond. Does that make sense?
Well, it turns out that if I want to, I can easily rotate this bond because remember, it's not locked in place with p orbitals; it's just an s orbital, so it's easy to twist it. And once it rotates, it's going to look different. Now if I draw my fence again, I would see that my big groups are on the same side. So this is what's called the s cis conformation. It turns out that molecules are constantly moving back and forth between these different types of conformations. So even though we draw hexane like this, it doesn't always look like that. Many times it's going to look like that and all the other single bonds are able to rotate too. So you can imagine, hexane actually doesn't always look like the zig-zag. Sometimes it's a little more crumpled up or whatever. These alternate arrangements, the fact that I have 2 different positions that my hexane could be in, are called conformers. Does that make sense so far? So that's the idea behind a conformer.