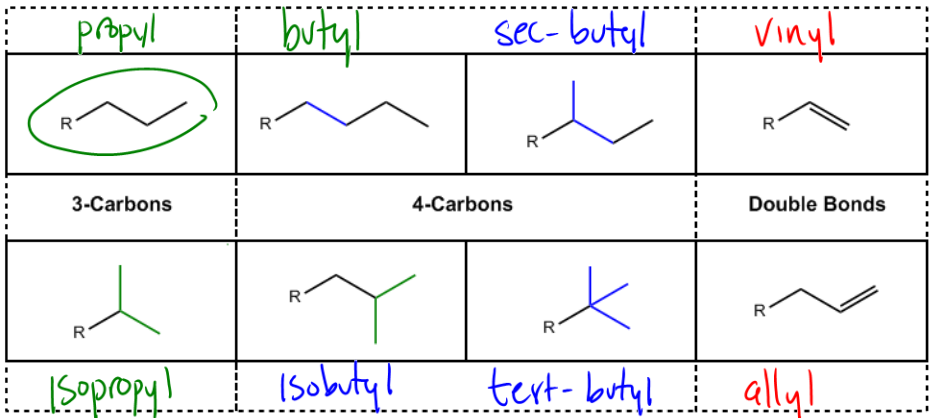
All right, guys. So now that we know how to name simple alkanes, we're going to start increasing the difficulty level slowly. Alright? So the first thing that I want to talk about is substituents. What happens if you have a substituent that you don't recognize the name for? And that's what this next section is about. Okay? So it turns out that even though we try to use the IUPAC system as much as we can, there are a few common substituents that remember common means that it's an old name. This is a name that comes from predates 1919, predates the convention. Okay? And these are names that have just stuck because they've been used for so long that even though they don't follow IUPAC convention, you're still supposed to know them. Okay. So just need to know them. So let's talk about the first two, and these are going to be really easy. The first one is just if I have a 3 carbon chain where the R group, R meaning the actual main chain, is attached to just the first carbon of the chain, this would be called propyl. Okay, so you already know this actually. Okay. So actually this part is the IUPAC part. The one over here is IUPAC. Alright? So don't worry about this one. This one you already know. Okay?
There's another one that you're supposed to know, which is how to have a 4 carbon chain. If I have a 4 carbon chain and it's attached right there, then that one would be called butyl. Okay, so these are easy. Alright, propyl and butyl, that has to do with the IAPAC nomenclature. But then there are a few different ways that we can arrange these substituents. So imagine R, it's only attached to one other carbon if you don't count the R, it's only attached to one other carbon on that substituent. What if you attach it to the secondary position? K. Well, then this gets its own name because it's not truly a propyl group. Even though it has 3 carbons, it's attached in a different spot, so this one would be called isopropyl. Okay? And why is it called isopropyl? Well, because it's an isomer of the propyl substituent. But still, that does not follow, like I said, that's not an IUPAC name. That's just the name that someone a long time ago gave it because they were like, oh, there's an isomer of propyl. So isopropyl, the way that I think about it is it looks kind of like a Y, so if you see that, you know it's an isopropyl group. Alright?
Now butyl is even more confusing because butyl can arrange in actually four possibilities, and all of them are going to get their own names, which sucks. So here we go. Butyl is usually attached at the primary position. Okay? But what if instead of attaching at the primary position of this four-carbon chain, what if I attached it at the secondary position? Okay? Then what would we call it? Well, what we would call this is actually just in italics sec-butyl. And guess what the sec stands for? It stands for "secondary." Okay, so what we're saying here is that this is a butyl group that is attached to the secondary position instead of the primary position like normal. So then how about if instead of being attached to the secondary position, now it's attached to a tertiary position. Okay? Now remember, in all of these substituents, I'm talking about only the substituent, not the R group. Okay? So what if it's attached in a tertiary position? Well then this is going to be called tert-butyl. Okay? And those are 3 really important forms of butyl that you need to know: butyl, sec-butyl, and tert-butyl. Okay? Now it turns out that butyl has 4 carbons, so there's even one more possibility, which would be what if it's still attached at the primary position like normal, but then it has a Y at the end, kind of like isopropyl? Well then this is going to get its own name, and that one's going to be called isobutyl. So isobutyl looks like isopropyl except it has this extra CH2 in the middle. Alright. So those are our propyls and our butyls. They kind of make sense the way that I'm describing them. Hopefully, that will help you memorize them a little bit better. But at the end of the day, this is just simple recognition. You should be able to just know what is what, and you will need to know this for many tests, well into Orgo 2 even.
I also want to just say one thing, which is that some textbooks and some professors will use a different letter or use a different notation for propyl and butyl. Instead of just saying propyl and butyl, sometimes they'll say en propyl and n-butyl. Now honestly, I have no clue what the n stands for. I don't know what it is. All I know is that n means that it's a straight chain. Okay. So sometimes your professor will say butyl. Sometimes, your professor says n-butyl. They're the same exact thing. N-butyl just says, hey, this is not isobutyl, this is not sec-butyl, this is a straight chain butyl. Alright? Cool. Awesome. So now there's just 2 more that I want you guys to know, and those are with double bonds. Alright? So what if you have a double bond coming straight off of your main chain? So your main chain is really long, you have a double bond coming right off of it. This gets its own unique name that's called vinyl. Okay? And you have heard of vinyl before? Vinyl is a plastic. That plastic is made out of these vinyl double bonds. Okay? So the fact that old records are called vinyls actually has to do with this molecule. But the molecule was named vinyl before the actual record was named a vinyl. Okay?
So then let's see, what if you have a double bond, but you're not attached directly to the R group instead you have a CH2 in between. Okay? So it's like a vinyl but has an extra CH2. This is going to be called, let me move out of the way, an allyl. Okay? Now I understand these are weird words. These are not words that are fun to remember. But honestly, there's no way to get around it. You just need to know them. Okay? So what I want to do now is do some more practice with IUPAC nomenclature, now knowing the names of these common substituents. Okay? So what we try to do is remember, we're still going to follow all the same rules as before. The only difference is that now if we get a substituent that looks like that at the end, now we're going to know the name for it. Okay? So I want to do as just a free response problem, like we're going to do it together. So go ahead and try to solve it and then when you're done, go ahead and go to the next video.