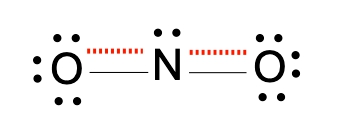
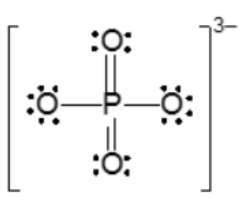
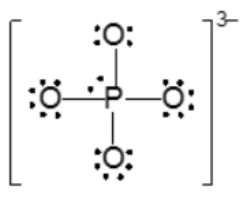
Now with resident structures, we're going to say a set of two or more valid Lewis dot structures for polyatomic ions possessing at least one π bond. Now we're going to say in a resonant structure we have the movement of only electrons from either a π bond or a lone pair here. This depicts two different ways that we can show our nitride ion. Here the π bond could be either on the left oxygen or it could be on the right oxygen. Both are valid depictions of the nitride ion.
Now here to show resonance structures we utilize double sided arrows. They're used to show that the resonance structures are equal or equivalent with each other. At this level of chemistry, we're going to say that they're equivalent. But once you get to higher levels of chemistry, such as organic chemistry, you're later learn that not all resonance structures are equivalent to one another.
Now we're going to say the real structure is represented by the composite of the resonance structures, and it's called the resonance hybrid. Now the resonance hybrid is the composite of all major resonance structures. When we say composite, we really mean an average of the two resonant structures. To draw the resonance hybrid, we place a dotted line anywhere a π bond has been. So there was a π bond on the left oxygen, so we do a dotted line there. There was a double bond on the right oxygen, so we show a dotted line there. So this would represent the resonance hybrid of our nitrite ion.