2. Atoms & Elements
Isotopes
2. Atoms & Elements
Isotopes - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
Isotopes are atoms of an element that have the same atomic number, but different mass number.
The Atomic View of Isotopes
1
concept
Isotopes
Video duration:
4mPlay a video:
Isotopes are elements with the same number of protons, but different number of neutrons.
2
example
Isotopes Example 1
Video duration:
3mPlay a video:

3
Problem
ProblemWhich of the following answers give the correct number of subatomic particles for 
A
38 protons, 38 neutrons and 38 electrons
B
88 protons, 50 neutrons and 50 electrons
C
38 protons, 50 neutrons and 38 electrons
D
38 protons, 27 neutrons and 13 electrons
4
Problem
ProblemWhich of the following answers give the correct number of subatomic particles for 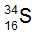
A
34 protons, 34 neutrons and 34 electrons
B
50 protons, 18 neutrons and 18 electrons
C
16 protons, 18 neutrons and 16 electrons
D
17 protons, 17 neutrons and 17 electrons
5
Problem
ProblemFill in the gaps for the following table of atoms.
Video duration:
5mPlay a video:
Was this helpful?
Do you want more practice?
We have more practice problems on Isotopes
Additional resources for Isotopes
PRACTICE PROBLEMS AND ACTIVITIES (57)
- Indicate the number of protons and neutrons in the following nuclei: (b) 193Tl.
- Indicate the number of protons and neutrons in the following nuclei: (c) neptunium-237.
- Consider an atom of 32P. (d) Are either of the atoms obtained in parts (b) and (c) isotopes of 32P? If so whic...
- Consider an atom of 58Ni. (c) What is the symbol for the isotope of 58Ni that possesses 33 neutrons?
- (b) What is the identity of the element whose isotopes you have selected?
- (a) Which two of the following are isotopes of the same element: 10646X, 10746X, 10747X?
- Write the correct symbol, with both superscript and subscript, for each of the following. (d) the isotope of ...
- Write the correct symbol, with both superscript and subscript, for each of the following. Use the list of elem...
- Write the correct symbol, with both superscript and subscript, for each of the following. Use the list of elem...
- In the following drawings, red spheres represent protons, and blue spheres represent neutrons. Which of the dr...
- Write isotopic symbols in the form X-A (e.g., C-13) for each isotope. d. the hydrogen isotope with one neutr...
- Write isotopic symbols in the form X-A (e.g., C-13) for each isotope. c. the uranium isotope with 146 neutrons
- Write isotopic symbols in the form X-A (e.g., C-13) for each isotope. b. the silver isotope with 62 neutrons
- Write isotopic symbols in the form X-A (e.g., C-13) for each isotope. a. the silver isotope with 60 neutrons
- Write isotopic symbols in the form AZX for each isotope. c. the potassium isotope with 21 neutrons
- Write isotopic symbols in the form AZX for each isotope. b. the copper isotope with 36 neutrons
- Write isotopic symbols in the form AZX for each isotope. a. the copper isotope with 34 neutrons
- Determine the number of protons and the number of neutrons in each isotope. d. 208 82Pb
- Determine the number of protons and the number of neutrons in each isotope. a. 14 7N
- Determine the number of protons and the number of neutrons in each isotope. a. 40 19K b. 226 88Ra c. 99 43T...
- The table to the right gives the number of protons (p) and neutrons (n) for four isotopes. (a) Write the symbo...
- The natural abundance of 3He is 0.000137%. (b) Based on the sum of the masses of their subatomic particles, wh...
- The natural abundance of 3He is 0.000137%. (a) How many protons, neutrons, and electrons are in an atom of 3He...
- The element argon has three naturally occurring isotopes, with 18, 20, and 22 neutrons in the nucleus, respect...
- The first atoms of seaborgium (Sg) were identified in 1974. The longest-lived isotope of Sg has a mass number ...
- The radioactive isotope cesium-137 was produced in large amounts in fallout from the 1985 nuclear power plant ...
- Write symbols for the following isotopes: (a) Z = 58 and A = 140 (b) Z = 27 and A = 60
- Nuclei with the same number of neutrons but different mass numbers are called isotones. Write the symbols of f...
- How many protons, neutrons, and electrons are in each of the following atoms? (d)
- How many protons, neutrons, and electrons are in each of the following atoms? (c)
- How many protons, neutrons, and electrons are in each of the following atoms? (b)
- How many protons, neutrons, and electrons are in each of the following atoms? (a)
- How many protons and neutrons are in the nucleus of the following atoms? (d)
- How many protons and neutrons are in the nucleus of the following atoms? (c)
- How many protons and neutrons are in the nucleus of the following atoms? (b)
- How many protons and neutrons are in the nucleus of the following atoms? (a)
- Which of the following isotope symbols can't be correct? (a) (b) (c) (d)
- Fluorine occurs naturally as a single isotope. How many protons, neutrons, and electrons are present in deuter...
- Lithium has 3 protons. How many neutrons are in the isotope Lithium-8?
- What is the mass number of the isotope with the symbol 3717Cl?
- Which of these refers to atoms with the same atomic number but different atomic masses?
- The atomic number of fluorine is 9 and its atomic mass is 19. How many neutrons does fluorine have?
- One isotope of sodium has 11 protons and 7 neutrons. What is the mass number of this isotope?
- Which pair of atoms constitutes a pair of isotopes of the same element?
- The oxygen isotope with 8 neutrons
- What is the mass number of the isotope with the symbol 37Cl17?
- Lithium has 3 protons. How many neutrons are in the isotope Lithium-8?
- Atoms of the same element, zinc for example, have the same number of
- How many protons, neutrons, and electrons are there in a neutral atom of 131I (iodine-131)?
- Two isotopes of the same element will have the same number of _____ but a different number of ____.
- How many protons, neutrons, and electrons are there in a neutral atom of 30P (phosphorus-30)?
- An isotope of yttrium has 39 protons and 59 neutrons. What is the mass number of that isotope?
- The superscript preceding each hydrogen atomic symbol (H) represents which of the following?
- All of the atoms making up any given element have the same number of ________.
- How many protons, neutrons, and electrons make up an atom of nitrogen-15?
- Can two atoms with the same mass number ever be isotopes of each other? Explain.
- Can two atoms with the same mass number ever be isotopes of each other?