8. Thermochemistry
Enthalpy of Formation
8. Thermochemistry
Enthalpy of Formation - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
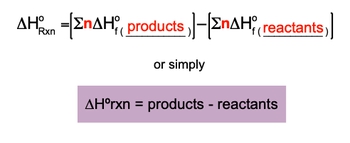
The Enthalpy of Formation for an element is a key component in determining the enthalpy of reaction.
Enthalpy of Formation
1
concept
Enthalpy of Formation
Video duration:
2mPlay a video:
2
example
Enthalpy of Formation Example 1
Video duration:
3mPlay a video:
3
Problem
ProblemThe oxidation of ammonia is illustrated by the following equation:
Calculate the enthalpy of reaction, ΔHRxn, based on the given standard heats of formation.

A
-906 kJ
B
-1273.2 kJ
C
1089.6 kJ
D
-183.6 kJ
4
Problem
ProblemConsider the following equation:
2 ClF3(g) + 2 NH3(g) → 1 N2(g) + 6 HF (g) + 6 Cl2(g) ΔHrxn = –1196 kJ
Determine the standard enthalpy of formation for chlorine trifluoride, ClF3.
A
-175.1 kJ
B
350.2 kJ
C
442.0 kJ
D
-1638 kJ
Do you want more practice?
We have more practice problems on Enthalpy of Formation
Additional resources for Enthalpy of Formation
PRACTICE PROBLEMS AND ACTIVITIES (90)
- A table of standard enthalpies of formation (ΔH°f) gives a value of −467.9 kJ/mol for NaNO3(s). Which reaction...
- What is ΔH for the explosion of nitroglycerin? (LO 9.14) 2 C3H5(NO3)3(l) → 3 N2(g) + 1/2 O2(g) + 6 CO2(g) + 5 ...
- Atomic hydrogen (H) is used in welding (AHW). The atoms recombine to hydrogen molecules with a large release o...
- Consider the following equilibrium between oxides of nitrogen 3 NO1g2 Δ NO21g2 + N2O1g2 (a) Use data in Append...
- Methanol 1CH3OH2 can be made by the reaction of CO with H2: CO1g2 + 2 H21g2 Δ CH3OH1g2 (a) Use thermochemical ...
- Acetylene 1C2H21g22 is used for welding because oxyacetylene is the hottest burning common fuel gas. Using sta...
- The fuel in high-efficiency natural-gas vehicles consists primarily of methane 1CH42. (a) How much heat is pro...
- Using values from Appendix C, calculate the value of H for each of the following reactions: (a) CaO1s2 + 2HF...
- Zinc metal reacts with hydrochloric acid according to the balanced equation: Zn(s) + 2 HCl(aq)¡ZnCl2(aq) + H2(...
- Complete combustion of 1 mol of acetone 1C3H6O2 liberates 1790 kJ: C3H6O1l2 + 4 O21g2 ¡ 3 CO21g2 + 3 H2O1l2 H°...
- Calcium carbide 1CaC22 reacts with water to form acetylene 1C2H22 and Ca1OH22. From the following enthalpy of ...
- Gasoline is composed primarily of hydrocarbons, including many with eight carbon atoms, called octanes. One o...
- Ethanol 1C2H5OH2 is blended with gasoline as an automobile fuel. (d) Calculate the mass of CO2 produced per...
- Ethanol 1C2H5OH2 is blended with gasoline as an automobile fuel. (c) Calculate the heat produced per liter of...
- Methanol 1CH3OH2 is used as a fuel in race cars. (b) Calculate the standard enthalpy change for the reaction,...
- Hydrazine (N2H4) is a fuel used by some spacecraft. It is normally oxidized by N2O4 according to the equation:...
- Pentane (C5H12) is a component of gasoline that burns according to the following balanced equation: C5H12(l ) ...
- Use standard enthalpies of formation to calculate ΔH °rxn for each reaction. d. Cr2O3(s) + 3 CO( g)¡2 Cr(s) ...
- Use standard enthalpies of formation to calculate ΔH °rxn for each reaction. c. 3 NO2( g) + H2O(l )¡2 HNO3(a...
- Use standard enthalpies of formation to calculate ΔH °rxn for each reaction. a. C2H4( g) + H2( g)¡C2H6( g)
- Use standard enthalpies of formation to calculate ΔH °rxn for each reaction. a. 2 H2S( g) + 3 O2( g)¡2 H2O(l )...
- Use standard enthalpies of formation to calculate ΔH °rxn for each reaction. b. SO2( g) + 12 O2( g)¡SO3( g)
- Ethanol (C2H5OH) can be made from the fermentation of crops and has been used as a fuel additive to gasoline. ...
- Top fuel dragsters and funny cars burn nitromethane as fuel according to the balanced combustion equation: 2 C...
- The explosive nitroglycerin (C3H5N3O9) decomposes rapidly upon ignition or sudden impact according to the bala...
- Methanol (CH3OH) has been suggested as a fuel to replace gasoline. Find ΔH °rxn, and determine the mass of car...
- All the oxides of nitrogen have positive values of ΔGf° at 298 K, but only one common oxide of nitrogen has a ...
- The standard enthalpies of formation of gaseous propyne 1C3H42, propylene 1C3H62, and propane 1C3H82 are +18...
- The standard enthalpies of formation of gaseous propyne 1C3H42, propylene 1C3H62, and propane 1C3H82 are +185....
- It is interesting to compare the 'fuel value' of a hydrocarbon in a hypothetical world where oxygen is not the...
- What is a compound's standard heat of formation?
- Use standard enthalpies of formation to calculate the standard change in enthalpy for the melting of ice. (The...
- Calculate ∆H°f in kJ/mol for benzene, C6H6, from the following data: 2 C6H6(l) + 15 O2(g) → 12 CO2(g) + 6 H2O(...
- Acetylene 1C2H22 and nitrogen 1N22 both contain a triple bond, but they differ greatly in their chemical prop...
- Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. To compare the energy of t...
- Hydrogen and methanol have both been proposed as alternatives to hydrocarbon fuels. Which fuel contains the mo...
- Acetic acid (CH3CO2H), whose aqueous solutions are known as vinegar, is prepared by reaction of ethyl alcohol ...
- Styrene (C8H8), the precursor of polystyrene polymers, has a standard heat of combustion of -4395 kJ/mol. Writ...
- Under certain nonstandard conditions, oxidation by O2( g) of 1 mol of SO2( g) to SO3( g) absorbs 89.5 kJ. The ...
- Methyl tert-butyl ether (MTBE), C5H12O, a gasoline additive used to boost octane ratings, has ΔH°f = -313.6 kJ...
- Methyl tert-butyl ether (MTBE) is prepared by reaciton of methanol (l) (ΔH°f = -239.2 kJ/mol) with 2-methyl-pr...
- Given the standard heats of formation shown in Appendix B, what is ΔH° in kilojoules for the reaciton CaCO3(s)...
- The ΔH for the oxidation of sulfur in the gas phase to SO3 is -204 kJ/mol and for the oxidation of SO2 to SO3 ...
- Given the standard heats of formation shown in Appendix B, what is ΔH° in kilojoules for the reaciton 3 N2O4(g...
- When magnesium metal is burned in air (Figure 3.6), two products are produced. One is magnesium oxide, MgO. T...
- Calcualte ΔH° in kilojoules for the synthesis of lime (CaO) from limestone (CaCO3), the key step in the manufa...
- Use the information in Table 9.2 to calculate ΔH° in kilojoules for the photosynthesis of glucose (C6H12O6) an...
- Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: ...
- Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: ...
- Use the data in Appendix B to find standard enthalpies of reaction in kilojoules for the following processes: ...
- Isooctane, C8H18, is the component of gasoline from which the term octane rating derives (b) The standard mola...
- The mechanism for the oxidation of HBr by O2 to form 2 H2O and Br2 is shown in Exercise 14.74. (a) Calculate t...
- Consider the following acid-neutralization reactions involving the strong base NaOH(aq): HNO31aq2 + NaOH1aq2¡N...
- The methane molecule, CH4, has the geometry shown in Figure 2.17. Imagine a hypothetical process in which the ...
- One of the best-selling light, or low-calorie, beers is 4.2% alcohol by volume and a 355-mL serving contains 1...
- Metallic mercury is obtained by heating the mineral cinnabar (HgS) in air: HgS1s2 + O21g2 S Hg1l2 + SO21g2 (a)...
- Methanol 1CH3OH2 is made industrially in two steps from CO and H2. It is so cheap to make that it is being con...
- Methanol 1CH3OH2 is made industrially in two steps from CO and H2. It is so cheap to make that it is being con...
- When a gaseous compound X containing only C, H, and O is burned in O2, 1 volume of the unknown gas reacts with...
- Combustion analysis of 0.1500 g of methyl tert-butyl ether, an octane booster used in gasoline, gave 0.3744 g...
- Acid spills are often neutralized with sodium carbonate or sodium hydrogen carbonate. For neutralization of ac...
- (b) Use the data in Appendix B to calculate ΔH° for the reaction of potassium metal with water.
- Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g) given the following reactions and enthalpies o...
- What is the isobaric heat of combustion for ethane, C2H6(g), in kJ per mole of ethane at 298.15 k?
- How much energy is required to decompose 765 g of PCl3
- What is the heat of combustion of ethane, C2H6, in kilojoules per mole of ethane? Enthalpy of formation values...
- Use standard enthalpies of formation to calculate δh∘rxn for the following reaction: 2 H2S(g) + 3 O2(g)→ 2 H2O...
- Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g)
- Use the molar bond enthalpy data in the table to estimate the value of δ𝐻°rxn for the equation C2H4(g) + HBr(...
- Use standard enthalpies of formation to calculate δh∘rxn for the following reaction: Cao(s) + CO2(g) → CaCO3(s...
- Calculate the enthalpy change for the reaction P4O6(s)+2 O2(g) → P4O10(s)
- For which one of the following is the enthalpy of the reaction the same as the enthalpy of formation?
- Use the molar bond enthalpy data in the table to estimate the value of δ𝐻∘rxn for the equation
- Use the values of δhf° in appendix 4 to calculate δh° for the following reactions.
- For which one of the following reactions is δh°rxn equal to the heat of formation of the product?
- Use the standard reaction enthalpies given below to determine δh°rxn for the following reaction:
- Calculate the heat evolved per mole on combustion of each substance to yield co2(g) and h2o(g).
- Calculate δs∘rxn for the following reaction. the δs∘ for each species is shown below the reaction.
- Calculate delta h for the following reaction
- Calculate the enthalpy of the reaction 2NO(g) + O2(g) → 2NO2(g) given the following reactions and enthalpies o...
- Using the table below, estimate δh for the "water splitting reaction": H2O(g) → H2(g) + 12 O2(g).
- Which answer best describes the transfer of heat that occurs when 1.20 mol of H2 reacts?
- Which answer best describes the transfer of heat that occurs when 1.70 mol of H2 reacts?
- Which answer best describes the transfer of heat that occurs when 1.40 mol of H2 reacts?
- Given the following at 25°C, calculate ΔHf for HCN(g) (in kJ/mol) at 25°C.
- In many ways, hydrogen as a biofuel compares very favorably to ethanol and other fuels.
- Which answer best describes the transfer of heat that occurs when 1.30 mol of H2 reacts?
- 4 NH3(g) + 5 O2(g) → 4 NO(g) + 6 H2O(g)
- What is the change in enthalpy associated with the combustion of 530 g of methane (CH4)?
- The enthalpy of the reaction is –2,840 kJ. What is the heat of combustion, per mole, of glucose?



