1. Intro to General Chemistry
Density of Non-Geometric Objects
1. Intro to General Chemistry
Density of Non-Geometric Objects - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
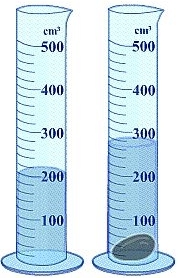
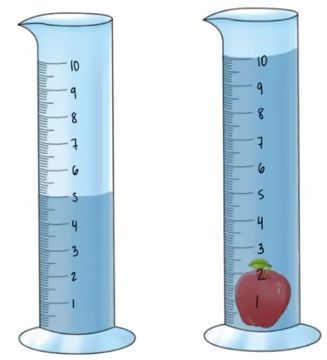
The density of non-geometric objects can be determined using water displacement.
Calculating Density
1
concept
Density of Non-Geometric Objects Concept and Example
Video duration:
3mPlay a video:
2
Problem
ProblemA piece of unknown solid weighs approximately 0.045 lbs. When a scientist places it in a glass beaker the water level increases from 200 mL to 260 mL. What is the density of the unknown solid in g/mL?
A
0.5 g/mL
B
0..2 g/mL
C
0.0007 g/mL
D
2.9 g/mL
E
0.3 g/mL
3
Problem
ProblemIf an irregularly shaped apple possesses a density of 0.96 g/cm3, what is its mass in milligrams? (The volume of the given cylinders are in mL).
A
4000 mg
B
5000 mg
C
0.0048 mg
D
4800 mg
E
5300 mg
Do you want more practice?
We have more practice problems on Density of Non-Geometric Objects
Additional resources for Density of Non-Geometric Objects
PRACTICE PROBLEMS AND ACTIVITIES (7)
- A 25.5 g sample of a metal was placed into water in a gradu-ated cylinder. The metal sank to the bottom, and t...
- A titanium bicycle frame displaces 0.314 L of water and has a mass of 1.41 kg. What is the density of the tita...
- A supposedly gold nugget displaces 19.3 mL of water and has a mass of 371 g. Could the nugget be made of gold...
- You would like to determine if a set of antique silverware is pure silver. The mass of a small fork was measur...
- A 32.65-g sample of a solid is placed in a flask. Toluene, in which the solid is insoluble, is added to the fl...
- Determine the density of an object that has a mass of 149.8 g and displaces 12.1 mL of water when placed in a ...
- Which property is used to determine if an object is made of pure silver (Ag)?