6. Chemical Quantities & Aqueous Reactions
Complete Ionic Equations
6. Chemical Quantities & Aqueous Reactions
Complete Ionic Equations - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
Complete Ionic Equations show aqueous compounds as fully dissociated ions.
Complete Ionic Equations
1
concept
Complete Ionic Equations
Video duration:
47sPlay a video:
The complete ionic equation shows all the aqueous compounds broken up into ions.
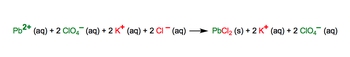
2
example
Complete Ionic Equations Example 1
Video duration:
2mPlay a video:
3
concept
Complete Ionic Equations
Video duration:
51sPlay a video:
Net Ionic Equation shows only the ions participating in the chemical reaction, without the spectator ions.
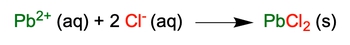
4
example
Complete Ionic Equations Example 2
Video duration:
4mPlay a video:
5
Problem
ProblemProvide the net ionic equation that occurs when the following aqueous compounds are mixed together:
Copper (II) Bromide and Lithium Hydroxide
Video duration:
4mPlay a video:
Was this helpful?
6
Problem
ProblemWhich of the following reagents could be used to separate the two anions from a solution containing magnesium nitrate and cesium hydroxide?
A
NH4CN
B
NaCl
C
KNO3
D
ZnBr2
E
CsBrO3
7
Problem
ProblemWhich of the following reagents could be used to separate the two cations from a solution containing Lead (IV) acetate and cesium permanganate?
A
Sr(NO3)2
B
TiC2H3O2
C
K2S
D
NaClO4
E
KNO3
Do you want more practice?
We have more practice problems on Complete Ionic Equations
Additional resources for Complete Ionic Equations
PRACTICE PROBLEMS AND ACTIVITIES (69)
- Which of the following ions will always be a spectator ion in a precipitation reaction? (a) Cl- (b) NO3- (c) N...
- Write a net ionic equation for the reaction that occurs when 10 mL of 0.5 M ammonium carbonate is mixed with 1...
- Which ions remain in solution, unreacted, after each of the following pairs of solutions is mixed? (a) potas...
- Which ions remain in solution, unreacted, after each of the following pairs of solutions is mixed? (c) ammon...
- Write balanced net ionic equations for the reactions that occur in each of the following cases. Identify the ...
- Write balanced net ionic equations for the reactions that occur in each of the following cases. Identify the s...
- Complete and balance the following molecular equations, and then write the net ionic equation for each: (b) Cu...
- Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (a)...
- Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (c)...
- Write the balanced molecular and net ionic equations for each of the following neutralization reactions: (b)...
- Write balanced molecular and net ionic equations for the following reactions, and identify the gas formed in e...
- Because the oxide ion is basic, metal oxides react readily with acids. (b) Based on the equation in part (a),...
- Write net ionic equations for the reactions listed in Problem 4.72.
- Write net ionic equations for the reactions listed in Problem 4.73.
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Write balanced complete ionic and net ionic equations for each reaction. b. MgS(aq) + CuCl2(aq)¡CuS(s) + MgC...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Predict whether a precipitation reaction will occur when aqueous solutions of the following substances are mix...
- Write balanced complete ionic and net ionic equations for each reaction. c. NaOH(aq) + HC2H3O2(aq)¡H2O(l ) +...
- Write balanced complete ionic and net ionic equations for each reaction. a. HCl(aq) + LiOH(aq)¡H2O(l ) + LiCl(...
- Write balanced complete ionic and net ionic equations for each reaction. d. HC2H3O2(aq) + K2CO3(aq)¡ H2O(l ) +...
- Write balanced complete ionic and net ionic equations for each reaction. b. NH4Cl(aq) + NaOH(aq)¡H2O(l ) + NH3...
- Mercury(I) ions (Hg22 + ) can be removed from solution by precipitation with Cl - Suppose that a solution cont...
- Lead(II) ions can be removed from solution by precipitation with sulfate ions. Suppose that a solution contain...
- Write balanced molecular and net ionic equations for the reaction between hydrobromic acid and potassium hydro...
- Write balanced molecular and net ionic equations for the reaction between nitric acid and calcium hydroxide.
- Write balanced complete ionic and net ionic equations for each acid–base reaction. b. HCHO2(aq) + NaOH(aq)¡
- Write balanced complete ionic and net ionic equations for each acid–base reaction. a. HI(aq) + RbOH(aq)¡
- The accompanying photo shows the reaction between a solution of Cd1NO322 and one of Na2S. (b) What ions remai...
- Antacids are often used to relieve pain and promote healing in the treatment of mild ulcers. Write balanced ne...
- Write balanced net ionic equations for the following reactions. (b)
- Write balanced net ionic equations for the following reactions. (a)
- Write balanced net ionic equations for the following reactions. Note that HClO3 is a strong acid. (a)
- Write balanced net ionic equations for the following reactions. Note that HClO3 is a strong acid. (b)
- Citric acid, C6H8O7, is a triprotic acid. It occurs naturally in citrus fruits like lemons and has application...
- (a) Write the net ionic equation for the reaction that occurs when a solution of hydrochloric acid (HCl) is m...
- A solution contains one or more of the following ions: Ag+ , Ca2 + , and Cu2 + . When you add sodium chloride ...
- A solution contains one or more of the following ions: Hg2 2 + , Ba2 + , and Fe2 + . When you add potassium ch...
- Write net ionic equations for the reactions that take place when aqueous solutions of the following substances...
- The reaction of MnO4- with oxalic acid (H2C2O4) in acidic solution, yielding Mn2+ and CO2 gas, is widely used ...
- Consider the redox titration (Section 4.13) of 120.0 mL of 0.100 M FeSO4 with 0.120 M K2Cr2O7 at 25 °C, assumi...
- What is the net ionic equation of the reaction of FeCl2 with NaOH?
- Give the complete ionic equation for the reaction (if any) that occurs when aqueous solutions of lithium sulfi...
- Enter the balanced complete ionic equation for NH4Cl(aq) + NaOH(aq) → H2O(l) + NH3(g) + NaCl(aq).
- Write the balanced net ionic equation for the reaction that occurs in the following case: Ba(NO3)2(aq) + K2SO4...
- Enter the net ionic equation representing aqueous acetic acid neutralized by aqueous barium hydroxide.
- Which ions remain in solution, unreacted, after each of the following pairs of solutions is mixed?
- Which of the following ions will always be a spectator ion in a precipitation reaction?
- As k2o dissolves in water, the oxide ion reacts with water molecules to form hydroxide ions.
- Which one of the following represents the net ionic equation for the reaction of HBr with Ca(OH)2?
- Write the net ionic equation for the reaction between hypochlorous acid and sodium hydroxide?
- What is the net ionic equation of the reaction of MgSO4 with Sr(NO3)2?
- What visible sign does not indicate a precipitation reaction when two solutions are mixed?
- What are the spectator ions in the reaction between KOH(aq) and HNO3(aq)?
- A student combines a solution of NaCl(aq) with a solution of AgNO3(aq), and a precipitate forms.
- What are the spectator ions in the reaction between Mg(OH)2 (aq) and HCl (aq)?
- Identify the solid product formed, if any, from the reaction of NH4NO3 and CaI2.
- What are the spectator ions in the reaction between KOH (aq) and HNO3 (aq)?
- What are the spectator ions in the reaction between KCl (aq) and AgNO3 (aq)?
- What is the net ionic equation of the reaction of MgSO4 with Ba(NO3)2?
- Write the net ionic equation (including phases) that corresponds to Zn(NO3)2
- What is the net ionic equation of the reaction of MgSO4 with Pb(NO3)2?
- Enter the net ionic equation, including phases, for the reaction of AgNO3(aq) with K2SO3(aq).
- Enter the balanced complete ionic equation for HCl(aq) + K2CO3(aq) → H2O(l) + CO2(g) + KCl(aq).

