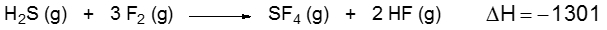11. Bonding & Molecular Structure
Bond Energy
11. Bonding & Molecular Structure
Bond Energy - Video Tutorials & Practice Problems

Get help from an AI Tutor
Ask a question to get started.
Bond Energy is the amount of energy stored within a chemical bond.
Bond Energy
1
concept
Bond Energy
Video duration:
1mPlay a video:
When individual bond energies are given, we will use the first formula.

2
example
Bond Energy Example 1
Video duration:
2mPlay a video:
3
Problem
ProblemConsider the following equation:
Determine the bond enthalpy value for the F–S bond.

A
-2301.0 kJ/mol
B
335.5 kJ/mol
C
-1171.5 kJ/mol
D
1301.0 kJ/mol
4
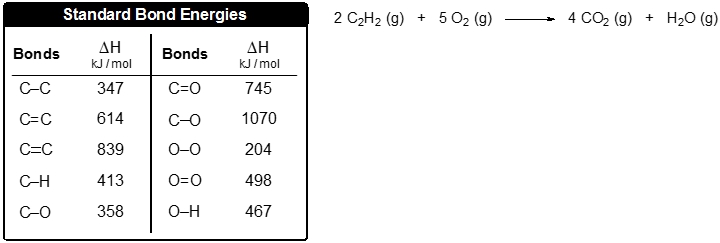
Problem
ProblemUse the bond energies to estimate the enthalpy of reaction for the combustion of 5 moles of acetylene:
A
-1074 kJ
B
2685 kJ
C
-2685 kJ
D
-430 kJ
Do you want more practice?
We have more practice problems on Bond Energy
Additional resources for Bond Energy
PRACTICE PROBLEMS AND ACTIVITIES (45)
- The graph shows how potential energy changes as a function of the distance between two atoms. (LO 7.2) What i...
- The following plot shows the potential energy of two Cl atoms as a function of the distance between them. (c) ...
- Isomers are molecules that have the same chemical formula but different arrangements of atoms, as shown here f...
- What is the best prediction for the carbon–oxygen bond length in the carbonate anion, CO3 2-? (LO 7.14) Data f...
- (b) Based on aver- age bond enthalpies, would you expect a photon capable of dissociating a C ¬ Cl bond to hav...
- Explain the difference in the bond dissociation energies for the following bonds: (C-F, 450 kJ/mol), (N-F, 270...
- State whether each of these statements is true or false. (e) The longer the bond, the more energy is stored ...
- Hydrogenation reactions are used to add hydrogen across double bonds in hydrocarbons and other organic compoun...
- Ethanol is a possible fuel. Use average bond energies to calculate ΔHrxn for the combustion of ethanol. CH3CH2...
- In the Chemistry and the Environment box on free radicals in this chapter, we discussed the importance of the ...
- Use bond enthalpies in Table 5.4 to estimate H for each of the following reactions: (a)
- Consider the collection of nonmetallic elements: B, As, O, and I. (b) Which two would form the longest single...
- The P¬P bond length in white phosphorus is 189 pm. The Cl¬Cl bond length in Cl2 is 199 pm. (b) What bond lengt...
- The P¬P bond length in white phosphorus is 189 pm. The Cl¬Cl bond length in Cl2 is 199 pm. (a) Based on these ...
- (a) The nitrogen atoms in an N2 molecule are held together by a triple bond; use enthalpies of formation in Ap...
- Consider the reaction 2 H21g2 + O21g2¡2 H2O1l2. (a) Use the bond enthalpies in Table 5.4 to estimate H for thi...
- Two compounds are isomers if they have the same chemical formula but different arrangements of atoms. Use Tabl...
- If hydrogen were used as a fuel, it could be burned according to this reaction: H2( g) + 1 2 O2( g)¡H2O( g) Us...
- If hydrogen were used as a fuel, it could be burned according to this reaction: H2( g) + 1 2 O2( g)¡H2O( g) Us...
- If hydrogen were used as a fuel, it could be burned according to this reaction: H2( g) + 1 2 O2( g)¡H2O( g) Wh...
- If hydrogen were used as a fuel, it could be burned according to this reaction: H2( g) + 1 2 O2( g)¡H2O( g) Wh...
- Calculate ΔHrxn for the combustion of octane (C8H18), a component of gasoline, by using average bond energies ...
- The heat of atomization is the heat required to convert a molecule in the gas phase into its constituent atoms...
- Calculate the heat of atomization (see previous problem) of C2H3Cl, using the average bond energies in Table ...
- Sulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur tetrafluoride monoxide 1OSF42 according to th...
- (a) Compare the bond enthalpies (Table 8.3) of the carbon– carbon single, double, and triple bonds to deduce a...
- Calculate an approximate heat of combustion for ethane (C2H6) in kilojoules by using the bond dissocation ener...
- (a) Use average bond enthalpies (Table 8.3) to estimate H for the atomization of naphthalene, C10H8:
- Use the data in Table 9.3 to calculate an approximate ∆H° in kilojoules for the synthesis of hydrazine from am...
- Use average bond enthalpies from Table 8.4 to estimate the enthalpies of the following gas-phase reactions: ...
- (b) When subjected to high pressure and heated, polyvinyl chloride converts to diamond. During this transforma...
- (a) In polyvinyl chloride shown in Table 12.6, which bonds have the lowest average bond enthalpy?
- The reaction S81g2 S 4 S21g2 has ΔH° = + 237 kJ (b) The average S ¬ S bond dissociation energy is 225 kJ/mol. ...
- The F ¬ F bond in F2 is relatively weak because the lone pairs of electrons on one F atom repel the lone pairs...
- Phosgene, COCl21g2, is a toxic gas used as an agent of warfare in World War I. (b) Using the table of bond dis...
- Ethanol is a possible fuel. use average bond energies to calculate δHrxn for the combustion of ethanol. CH3CH2...
- Calculate the average molar bond enthalpy of the carbon–hydrogen bond in a CH4 molecule.
- Use average bond energies to calculate δHrxn for the following hydrogenation reaction: H2C=CH2(g) + H2(g) → H3...
- Calculate δhrxn for the combustion of octane (C8H18) by using average bond energies.
- Use average bond enthalpies to estimate the enthalpy δhrxn of the following reaction: 2 SF4(g) + O2(g) → 2 OSF...
- Ethanol is a possible fuel. use average bond energies to calculate δHrxn for the combustion of ethanol.
- Predict whether the following reactions will be exothermic or endothermic.
- Calculate δh° for the reaction using the given bond dissociation energies.
- Ethanol is a possible fuel. Use average bond energies to calculate δHrxn for the combustion of ethanol. CH3CH2...
- 2 H3C−CH3(g) + 7O2(g) → 4 CO2(g) + 6 H2O(g)